
[ad_1]
Model 1.0 of the FDA PreSTAR template, launched March 29th, now allows using the PreSTAR template for 513g requests for info.
What’s a 513g request?
A “513g” is a request for classification info from the FDA. The reference is to a Meals, Drug & Beauty Act part. The aim of the submission is to ask the FDA what product classification can be most acceptable to your machine and what the suitable regulatory pathway will probably be. The regulation requires the FDA to offer a written response inside 60 days of receiving the 513g request. The submission additionally requires cost of an FDA person payment eligible for a small enterprise low cost.
Is it required to make use of the brand new FDA PreSTAR v1.0 template for a 513g request?
No, the FDA PreSTAR will not be required to submit a 513g request for info. The FDA has not up to date the 2019 steering doc but, and the FDA continues to permit using an FDA eCopy for 513g submissions. Nevertheless, the up to date PreSTAR template simplifies the method of submitting a request for classification info.
What’s the required content material of a 513g request?
Web page 15 of the FDA steering for 513g requests specifies the next content material:
- cowl letter,
- description of the machine,
- description of what the machine is for use for,
- any proposed labeling or promotional materials for the machine and, as relevant, any labeling or promotional materials of an analogous, legally marketed machine, if accessible.
The steering additionally particulars the minimal necessities for these 4 content material necessities. The quilt letter necessities specified within the steering embody “your particular query(s) in regards to the class during which a tool has been categorized and/or the regulatory necessities relevant to a tool.” When the PreSTAR is used for a pre-submission, there’s a designated part on the finish of the template for getting into questions. Nevertheless, v1.0 doesn’t enable this feature for a 513g. Subsequently, questions have to be added to the duvet letter as an alternative. The template Medical Machine Academy created for a 513g contains the next default query:
Purpose for the 513(g) Submission:
[Company Name] plans to submit a pre-market submission in 202x, and the corporate is requesting a choice from the FDA concerning the regulatory pathway for the topic machine.
This part might be modified to incorporate further questions, relying on the precise motive for the 513g request.
When ought to a 513g request for info be submitted?
Normally, machine firms ask me if I feel they need to submit a 513g or a pre-submission request to reply questions concerning the testing necessities. Usually, the machine has a recognized product classification code that requires a submission of 510(okay). Generally, there’ll even be a Particular Controls Steering doc accessible for the product classification. In these conditions, a 513g is solely pointless. I can perceive the difficulties folks expertise when navigating the FDA product classification database as a result of the database doesn’t use fashionable pure language search algorithms like Google. Nevertheless, a larger concern is that the majority firms ask this query after they’ve already began the event of their machine and earlier than they plan to provoke design verification testing. That is very late within the design course of, and it’s even a little bit late to conduct a pre-submission request. Your 513g submission needs to be in the course of the starting of your design challenge (i.e., in the course of the idea or feasibility phases of design) to confirm the proposed regulatory pathway.
Easy methods to put together a 513g
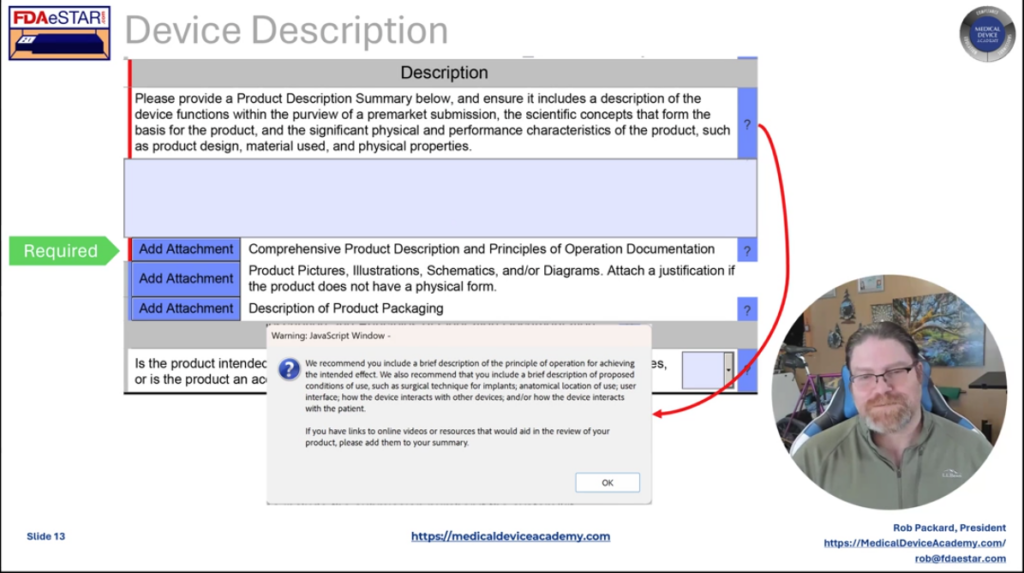
For any machine submission, together with a 513g, you need to put together an in depth machine description for the FDA. Many firms discover this troublesome. Subsequently, we offer a template for the machine description. Along with the machine description, we suggest together with a replica of the draft labeling and directions to be used (IFU) with every machine submission. A pre-submission doesn’t require draft labeling, however a 513g classification request does to make sure the FDA understands your supposed use for the machine. Subsequently, we offer templates for firms to arrange these drafts.
Different Sources
If you have to submit a 513g request, you possibly can study extra about FDA content material necessities by watching our 513g submission webinar. Additionally, you will obtain entry to our 513g templates if you buy the webinar bundle. We additionally present the templates for the machine description, draft label, and draft directions to be used (IFU) to new shoppers submitting a pre-submission assembly request, a 510k submission, or a De Novo Classification Request. As well as, there are six (6) different templates included with the 513g webinar bundle. These templates are particularly required for De Novo submissions, and we suggest together with them should you imagine your machine requires a De Novo submission.
[ad_2]
