
[ad_1]
A brand new model of ISO 17511 has been not too long ago revealed and on this article we’ll dig into the necessities of this ISO customary, centered on guaranteeing metrological traceability for In-vitro Diagnostic Medical Gadgets. We have now been already discussing about IVD gadgets, notably in relation to ISO 15189, ISO 23640 associated to the soundness of the IVD gadgets, efficiency analysis plan, and EU Reference laboratories in accordance with IVDR 2017/746.
Earlier than getting into into the detailed necessities of the usual, it is very important outline what does precisely imply metrological traceability. Based on the Worldwide vocabulary of metrology traceability is “a property of a measurement consequence that may be associated to a reference by means of a documented unbroken chain of calibrations, every contributing to the measurement uncertainty”.
It is usually deem needed to totally perceive the idea of calibration. Within the traceability context, Calibration means utilizing the worth of the earlier reference materials within the traceability hierarchy to assign worth by means of measurement to the subsequent calibrator within the hierarchy. Often that is usually performed by buying a licensed reference materials at two or extra applicable concentrations, which can be utilized for calibrating a linear measurement equation used for measurement.
Traceability Hierarchies Based on ISO 17511
ISO 17511 defines six calibration hierarchies and worth switch fashions, relying on the availablity of the traceability to SI.
Calibration within the traceability hierarchy is expounded to the likelihood to make use of the worth of the earlier reference materials within the traceability hierarchy to assign worth by means of measurement to the subsequent calibrator within the hierarchy.
The six materials tithing the calibration hierarchies are:
- Licensed major reference materials – ISO 15194 conforming;
- Main calibrator – ready as resolution of m. 1 in appropriate solvent;
- Secondary, commutable licensed reference materials conforming to ISO 15193. Matrix is pooled human plasma;
- Producers working calibration;
- Calibrator for the end-user measurement system;
- Human pattern with consequence
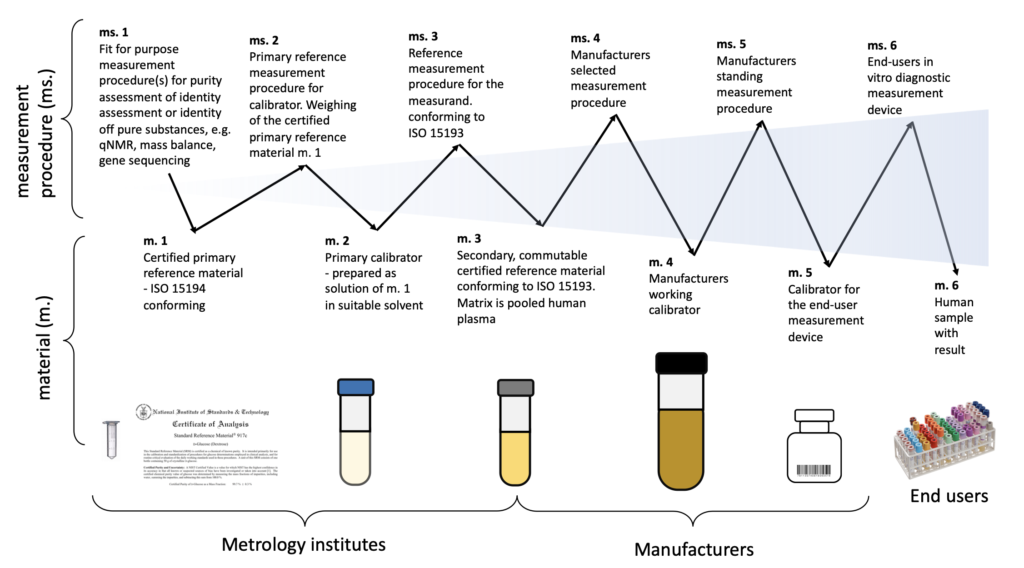
Licensed reference supplies are self-evident on the prime/starting of the traceability hierarchy and end-user calibrators additional on within the hierarchy.
The technical documentation of the calibration hierarchy wants to incorporate a determine or different illustration describing the linkage from the last word outcomes utilizing the human samples examined with the required measuring system as much as the best accessible metrological reference.
For every step within the calibration hierarchy, the amount being measured within the related reference materials or human samples, within the case of the ultimate measurements with a measuring system, should be recognized, and the connection between the measured amount (or portions) and the measurand should be established.
stipulations:
- Traceability to SI
- Availability of licensed reference supplies
- Availability reference measurement procedures
- Availability of harmonization protocols
The major reference supplies are normally extremely purified. They include a physiochemically well-defined analyte, evaluated for compositional stability integrity, and accompanied by a certificates (t – a licensed reference materials).
A major calibrator shall be ready from a major reference materials and value-assigned utilizing a major reference measurement process.
The worth to a secondary calibrator or secondary reference materials should be given by means of an applicable reference measurement process for the measurand.
Subscribe to QualityMedDev E-newsletter
QualityMedDev is an internet platform centered on High quality & Regulatory matters for medical system enterprise; Observe us on LinkedIn and Twitter to remain updated with most essential information on the Regulatory discipline.
QualityMedDev is without doubt one of the largest on-line platform supporting medical system enterprise for regulatory compliance matters. We offer regulatory consulting companies over a broad vary of matters, from EU MDR & IVDR to ISO 13485, together with threat administration, biocompatibility, usability and software program verification and validation and, on the whole, assist in preparation of technical documentation for MDR.
Our sister platform QualityMedDev Academy offers the likelihood to observe on-line and self-paced coaching programs centered on regulatory compliance matters for medical system. These coaching programs, developed in collaboration with extremely expert professionals within the medical system sector, permits you to exponentially enhance your competencies over a broad vary of high quality and regulatory matters for medical system enterprise operations.

Don’t hesitate to subscribe to our E-newsletter!
[ad_2]