[ad_1]
Study why it’s essential begin with software program validation documentation earlier than you soar into software program improvement.
At the least as soon as every week, I communicate with the founding father of a brand new MedTech firm that developed a brand new software program software as a medical gadget (SaMD). The founder will ask me to clarify the method for acquiring a 510(ok), they usually need assist with software program validation documentation. Many individuals I communicate with have by no means even heard of IEC 62304.
What are the 11 software program validation paperwork required by the FDA?
In 2005 the FDA launched a steerage doc outlining software program validation documentation content material required for a premarket submission. There have been 11 paperwork recognized in that steerage:

What the FDA steerage fails to clarify is that a few of these paperwork should be created earlier than software program improvement begins, or your software program validation documentation will likely be lacking vital design components. Subsequently, you will need to create a software program improvement plan that schedules actions that end in these paperwork on the proper time. In distinction, 4 of the eleven paperwork can wait till your software program improvement is full.
Which of the software program validation paperwork can wait till the top?
The extent of concern solely determines what paperwork the FDA needs to evaluate in a submission fairly than what paperwork are wanted for a design historical past file. Actually, the extent of concern (LOC) doc is now not required as a separate doc in premarket submissions utilizing the FDA eSTAR template as a result of the template already incorporates the questions that doc your LOC. The revision stage historical past doc is solely a abstract of revisions made to the software program through the improvement course of, and that doc might be created manually or routinely on the finish of the method, or the revision stage historical past is usually a residing doc that’s created as modifications are made. The traceability matrix can be a residing doc created as modifications are made, however its solely goal is to behave as a instrument to supply traceability from hazards to software program necessities, to design specs, and at last to verification and validation reviews. Different software program instruments, comparable to Software Lifecycle Administration (ALM) Software program, are designed to make sure the traceability of each hazard and requirement all through the complete improvement course of. Lastly, unresolved anomalies ought to solely be documented on the time of submission. The listing could also be incomplete till all verification and validation testing is accomplished, and the listing needs to be the shortest on the time of submission.
What documentation will likely be created close to the top of improvement?
The software program design specification (SDS) is usually a residing doc till your improvement course of is accomplished, and it’s possible you’ll must replace the SDS after the preliminary software program launch so as to add new options, keep interoperability with software program equipment, or change safety controls. The SDS can’t start, nonetheless, till you’ve software program necessities and the fundamental structure outlined. The verification and validation actions are discrete paperwork created after every revision of the SDS and should due to this fact be one of many final paperwork created–particularly when offered to the FDA as a abstract of the verification and validation efforts.
Which validation paperwork do you want first?
At the start of software program improvement, you want a process(s) that defines your software program improvement course of. That process ought to have a piece that explains the software program improvement surroundings–together with how patches and upgrades will likely be managed and launched. In case you don’t have a high quality system process that defines your improvement course of, then every developer could doc their coding and validation actions in another way. That doesn’t imply which you could’t enhance or change the process as soon as improvement has begun, however we suggest limiting the implementation of a revised process when making main software program modifications and discussing how revisions will likely be carried out for any work that continues to be in progress or has already been accomplished.
When do the remaining software program validation paperwork get created?
The remaining 4 software program validation paperwork required for a premarket submission to the FDA are:
- Software program description
- Software program hazard evaluation
- Software program necessities specification (SRS)
- Structure design chart
Your improvement course of will likely be iterative, and due to this fact, you have to be constructing and refining these 4 paperwork iteratively in parallel together with your software program code. At the start of your venture, your design plan will want a short software program description. Your preliminary software program description wants to incorporate the indications to be used, an inventory of the software program’s purposeful components, and the weather of your person specification (i.e., meant affected person inhabitants, meant customers, and person interface). In case you are utilizing lean startup methodology, the primary model of your gadget description will likely be restricted to a minimal viable product (MVP). The goal efficiency of the MVP needs to be documented as an preliminary software program necessities specification (SRS). This preliminary SRS would possibly solely consist of 1 requirement, however the SRS will increase rapidly. Subsequent, it’s essential carry out an preliminary software program hazard evaluation to establish the potential hazards. It is very important do not forget that software program hazards are usually hazardous conditions and will not be restricted to direct bodily hurt. For every potential hazard you establish in your hazard evaluation, you will have a software program requirement to deal with every hazard, and every requirement must be added to your SRS. As your software program turns into extra complicated by including software program options, your gadget description must be up to date. As you add features and necessities to your software program software, your SRS will want updates too. Lastly, your improvement staff will want a instrument to trace knowledge move and calculations from one software program perform to the subsequent. That instrument is your structure design chart, and you’ll want to manage your SRS to match the varied software program modules recognized in your structure diagram. This part is iterative and non-linear, you’ll at all times have failures, and usually a staff of builders will collaborate nearly. Sustaining a present model of the 4 software program paperwork is vital to conserving your improvement staff on monitor.
How do you carry out a software program hazard evaluation?
Some of the vital pre-requisite duties for software program builders is conducting a hazard evaluation. You’ll be able to develop an algorithm earlier than you write any code, however in the event you begin growing your software to execute an algorithm earlier than you carry out a software program hazard evaluation, you’ll be lacking vital software program necessities. Software program hazard evaluation is completely different from conventional gadget hazard evaluation as a result of software program hazards are distinctive to software program. A standard gadget hazard evaluation consists of three steps: 1) answering the 37 questions in Annex A of ISO/TR 24971:2020, 2) systematically figuring out hazards by utilizing Desk C1 in Annex C of ISO 14971:2019, and three) reviewing the dangers related to earlier variations of the gadget and related competitor gadgets. A software program hazard evaluation can have only a few hazards recognized from steps 1 and a pair of above. As an alternative, the very best useful resource for software program hazard evaluation is IEC/TR 80002-1:2009. You must nonetheless use the opposite two requirements, particularly if you’re growing software program in a medical gadget (SiMD) or firmware, however IEC/TR 80002-1 has a wealth of tables that can be utilized to populate your preliminary hazards evaluation and to replace your hazard evaluation if you add new options.
How do you doc your hazard evaluation?
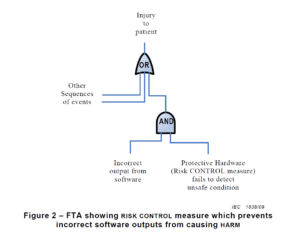
One other key distinction between a conventional hazard evaluation and a software program hazard evaluation is the way you doc the hazards. Most gadgets use a design FMEA (dFMEA) to doc hazards. The dFMEA is a bottom-up technique for documenting your danger evaluation by beginning with gadget failure modes. One other instrument for documenting hazards is a fault tree diagram.
A fault tree is a top-down technique for documenting your danger evaluation, the place you establish the entire potential causes that contribute to a particular failure mode. Fault tree diagrams lend themselves to criticism investigations as a result of criticism investigations start with the identification of the failure (i.e., criticism) on the prime of the diagram. For software program, the FDA is not going to help you use the chance of prevalence to estimate dangers. As an alternative, software program danger estimation needs to be restricted to the severity of the potential hurt. Subsequently, a fault tree diagram is mostly a greater instrument for documenting software program danger evaluation and organizing your listing of hazards. You would possibly even take into account making a separate fault tree diagram for every module of your software program recognized within the structure diagram. This method may even allow you to establish the potential influence of any software program hazard by wanting on the failure on the prime of the fault tree. The upper the potential severity of the software program failure, the extra assets the software program staff wants to use to growing software program danger controls and verifying danger management effectiveness for the related fault tree.
[ad_2]